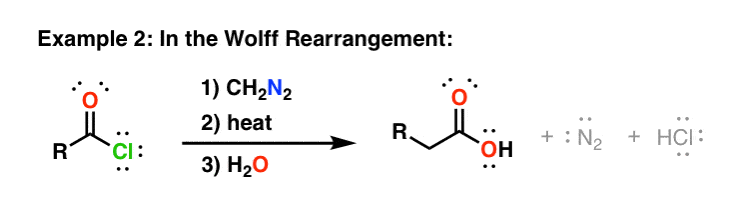
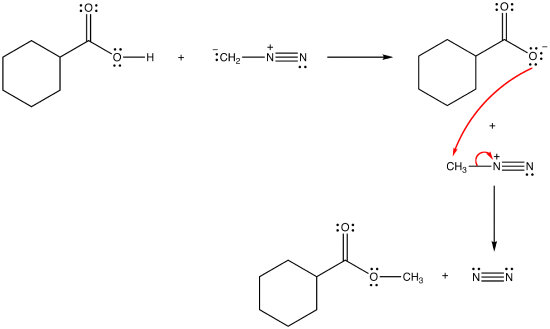
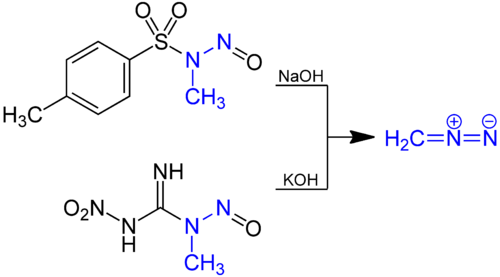
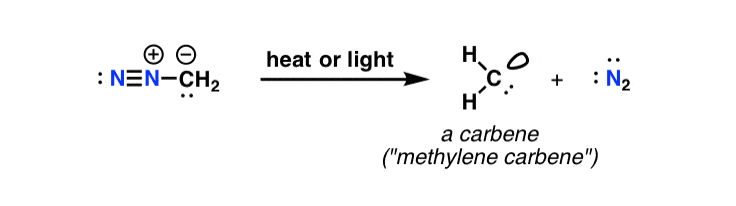
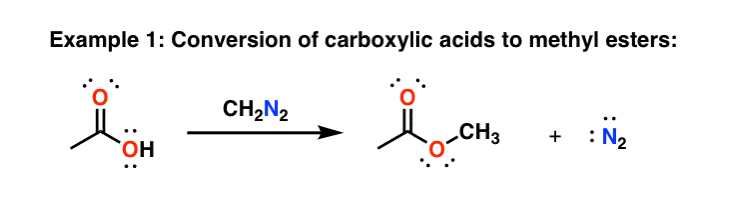
Reaction mechanism of the methylation of a carboxylic acid R-COOH with... | Download Scientific Diagram

Mechanism of Methyl Esterification of Carboxylic Acids by Trimethylsilyldiazomethane - Kühnel - 2007 - Angewandte Chemie International Edition - Wiley Online Library

Methyl esterification of fatty acids and eicosanoids with a novel reagent trimethylsilyldiazomethane for analysis by gas chromatography-mass spectrometry | Semantic Scholar
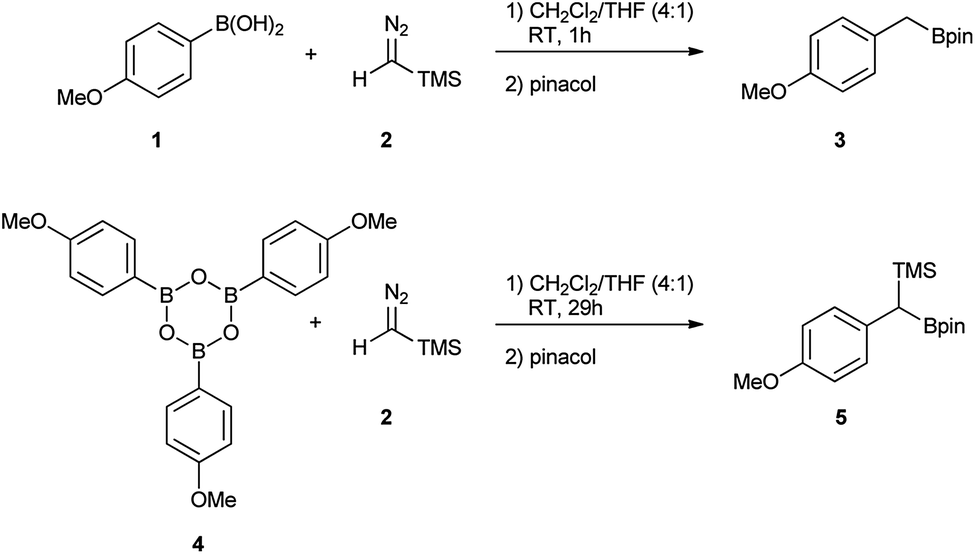
Unveiling the role of boroxines in metal-free carbon–carbon homologations using diazo compounds and boronic acids - Chemical Science (RSC Publishing) DOI:10.1039/C7SC02264F

The reaction of PtdIns(3,4,5)P3 with TMS-diazomethane. TMS-diazomethane... | Download Scientific Diagram

PDF) Trimethylsilyldiazomethane – A Mild and Efficient Reagent for the Methylation of Carboxylic Acids and Alcohols in Natural Products

Trimethylsilyldiazomethane: a safe non-explosive, cost effective and less-toxic reagent for phenol derivatization in GC applications. - Abstract - Europe PMC