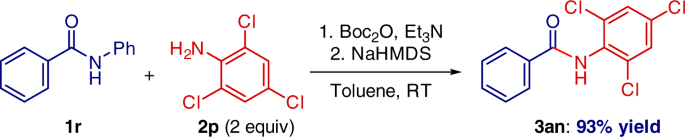
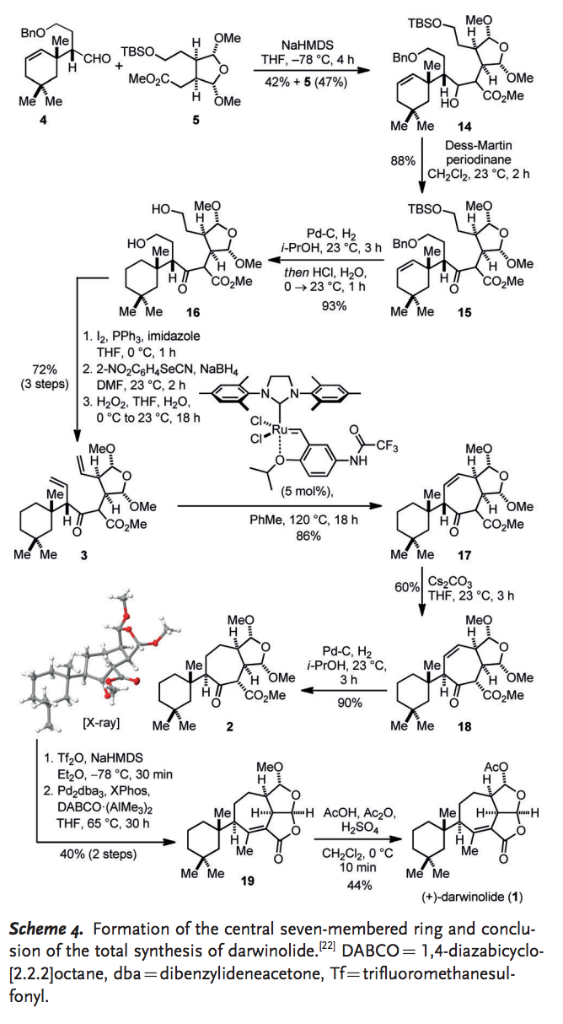
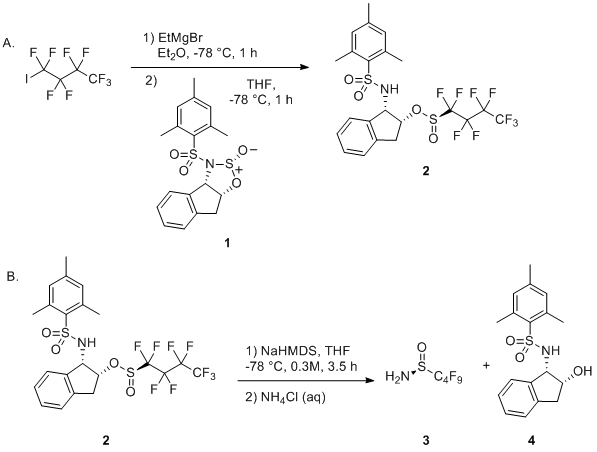
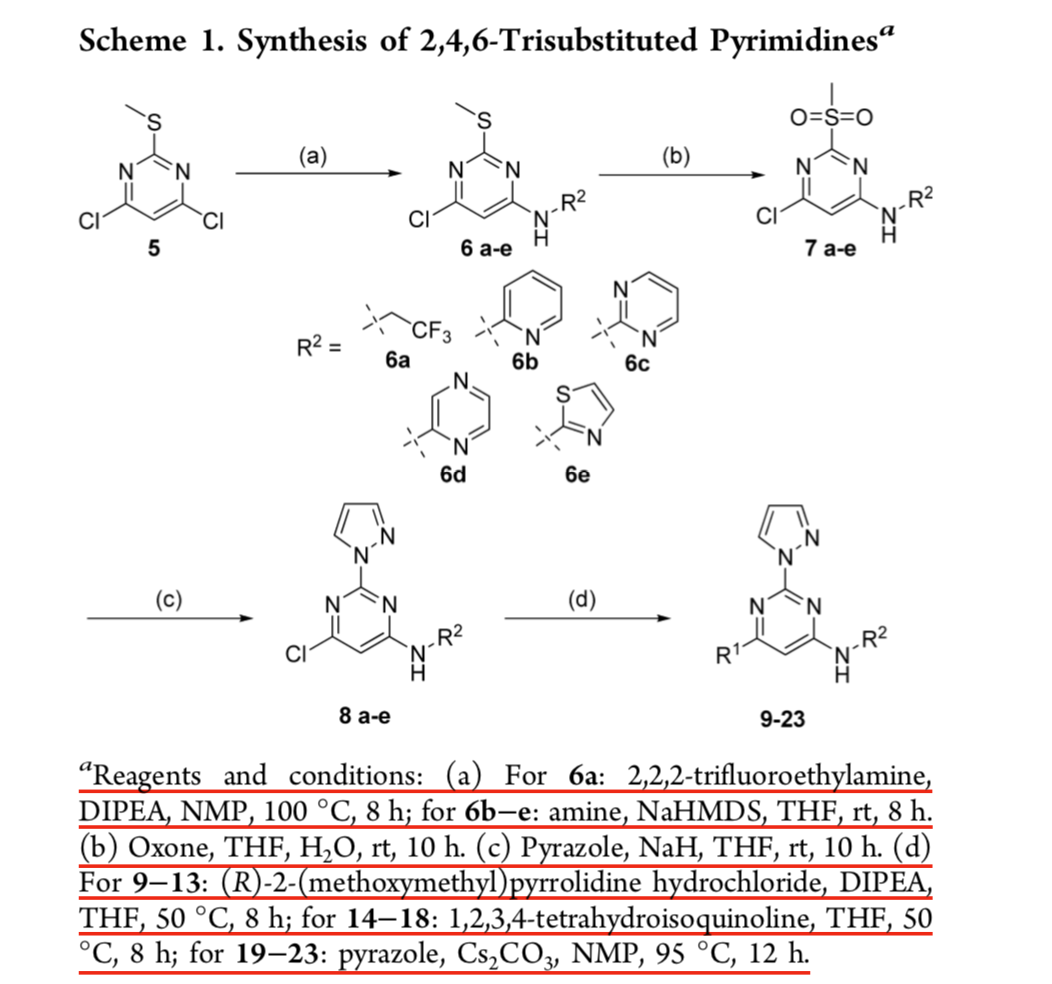
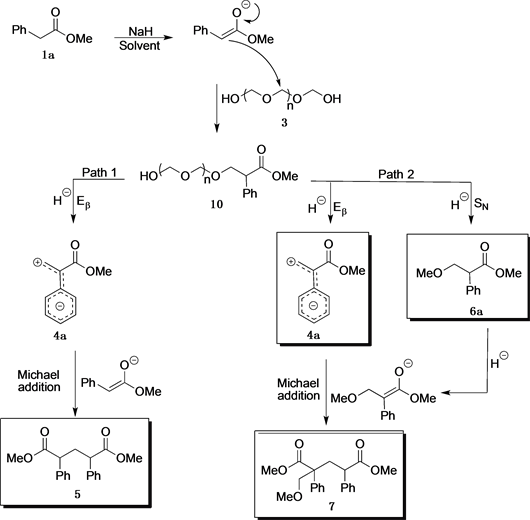
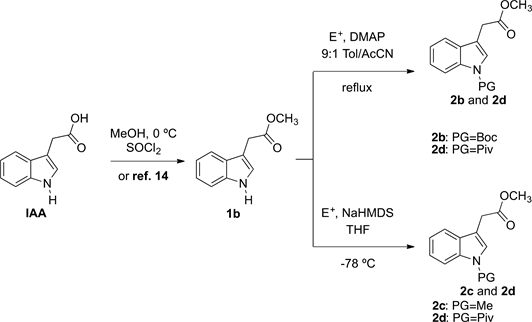
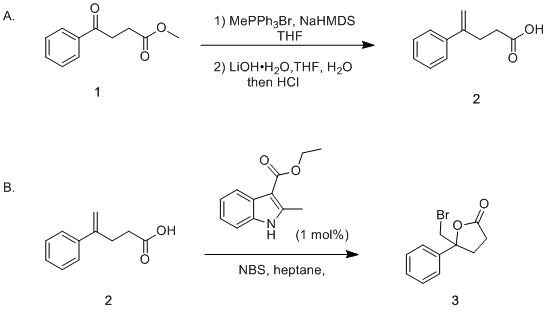
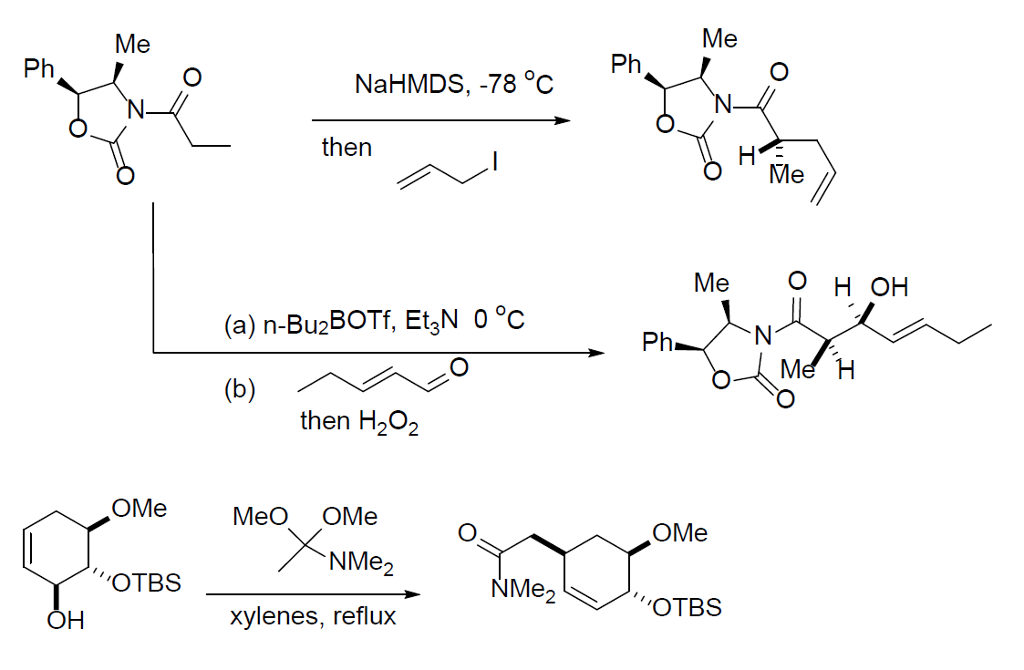
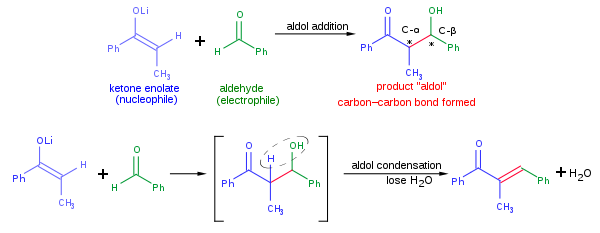
Base-promoted addition of DMA with 1,1-diarylethylenes: application to a total synthesis of (−)-sacidumlignan B - Organic & Biomolecular Chemistry (RSC Publishing)
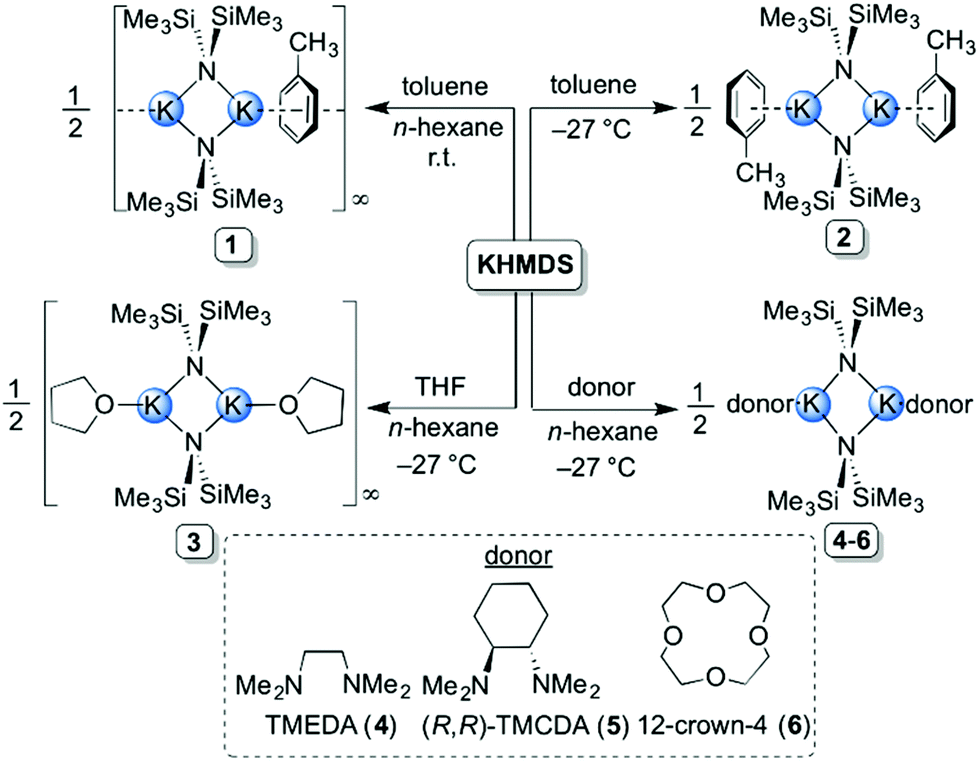
Exploring the solid state and solution structural chemistry of the utility amide potassium hexamethyldisilazide (KHMDS) - Dalton Transactions (RSC Publishing) DOI:10.1039/C7DT01118K

Catalysts | Free Full-Text | Transition Metal-Catalyzed α-Position Carbon–Carbon Bond Formations of Carbonyl Derivatives | HTML

6,7‐Benzotropolone Syntheses Based on Ring‐Closing Metatheses and Four‐Electron Oxidations - Kreibich - 2020 - European Journal of Organic Chemistry - Wiley Online Library

AlMe3‐Mediated Regio‐ and Chemoselective Reactions of Indole with Carbamoyl Electrophiles - Velavan - 2013 - European Journal of Organic Chemistry - Wiley Online Library
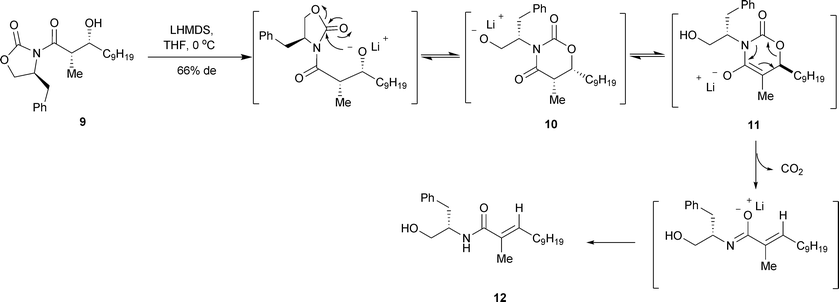
A temporary stereocentre approach for the asymmetric synthesis of chiral cyclopropane-carboxaldehydes - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B908600E

Synthesis of common intermediate 16 m-CPBA, meta-chloroperoxybenzoic... | Download Scientific Diagram

Anomalous Z-isomer content in Wittig reaction products from keto-stabilised ylides with ortho-heteroatom substituted benzaldehydes - ScienceDirect
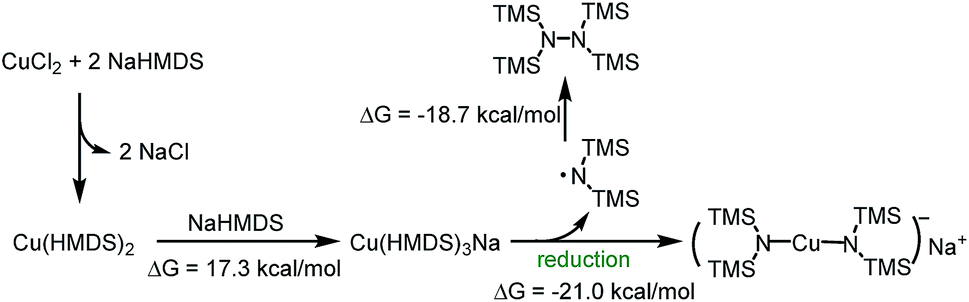
Revealing the reduction process of Cu(ii) by sodium bis(trimethylsilyl)amide - Faraday Discussions (RSC Publishing)