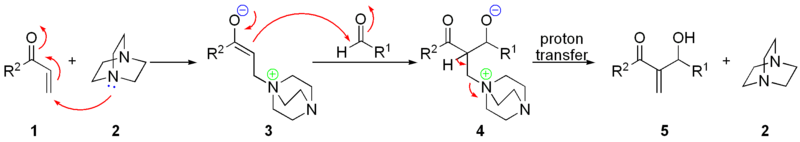
Scheme 3. Mechanism of Baylis-Hillman reaction of methyl acrylate and... | Download Scientific Diagram
![Proposed mechanism for the synthesis of tetrazolo[1,5-a]pyrimidine with... | Download Scientific Diagram Proposed mechanism for the synthesis of tetrazolo[1,5-a]pyrimidine with... | Download Scientific Diagram](https://www.researchgate.net/publication/318232004/figure/fig3/AS:961898610708493@1606345908272/Proposed-mechanism-for-the-synthesis-of-tetrazolo1-5-apyrimidine-with-DABCO_Q640.jpg)
Proposed mechanism for the synthesis of tetrazolo[1,5-a]pyrimidine with... | Download Scientific Diagram
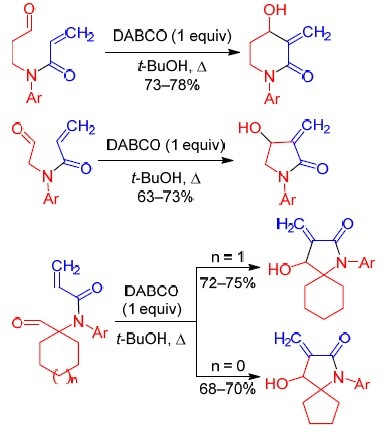
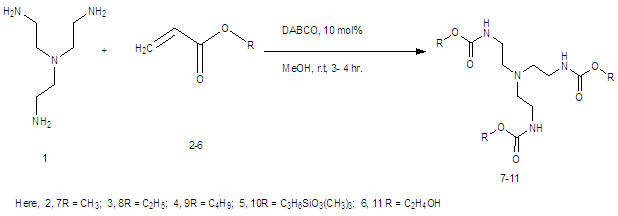
The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink
![A quaternary ammonium salt [H-dabco][AcO]: as a recyclable and highly efficient catalyst for the one-pot synthesis of β-phosphonomalonates - RSC Advances (RSC Publishing) DOI:10.1039/C5RA02743H A quaternary ammonium salt [H-dabco][AcO]: as a recyclable and highly efficient catalyst for the one-pot synthesis of β-phosphonomalonates - RSC Advances (RSC Publishing) DOI:10.1039/C5RA02743H](https://pubs.rsc.org/image/article/2015/RA/c5ra02743h/c5ra02743h-s3_hi-res.gif)
A quaternary ammonium salt [H-dabco][AcO]: as a recyclable and highly efficient catalyst for the one-pot synthesis of β-phosphonomalonates - RSC Advances (RSC Publishing) DOI:10.1039/C5RA02743H

The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties | SpringerLink

Unveiling the mechanism of N‐methylation of indole with dimethylcarbonate using either DABCO or DBU as catalyst - Vendramini - 2021 - Journal of Mass Spectrometry - Wiley Online Library
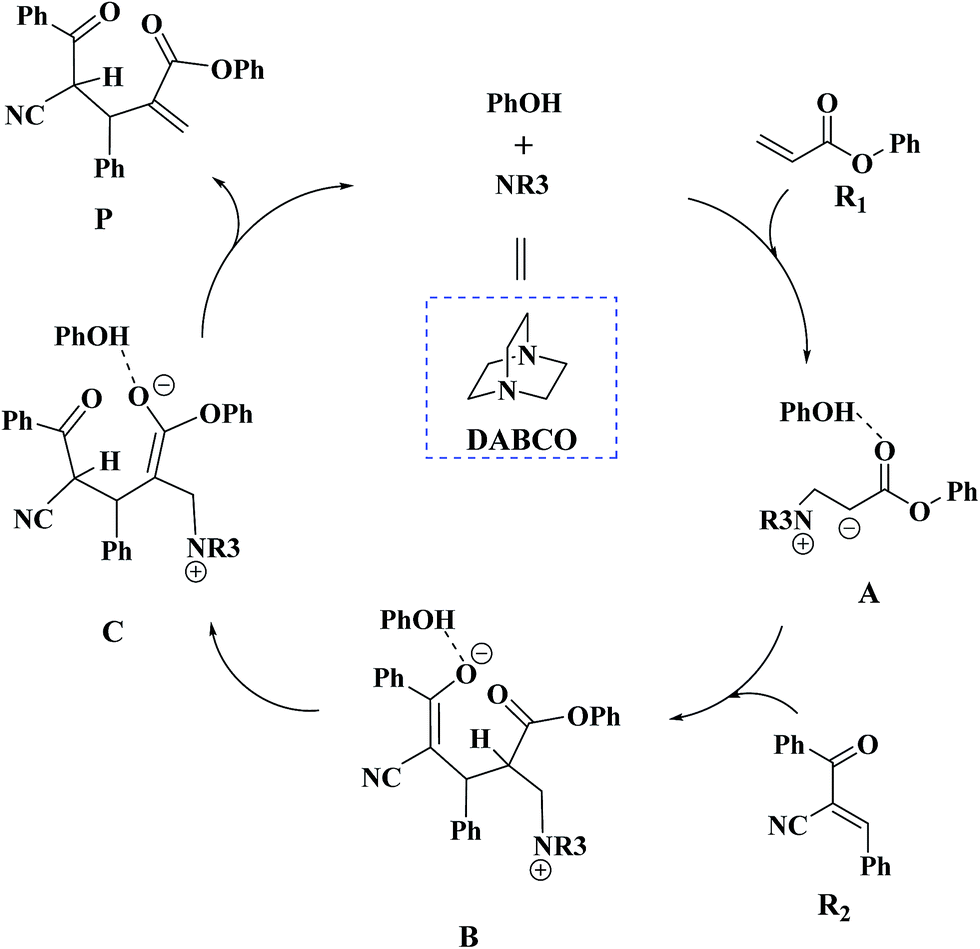
Mechanisms and stereoselectivities of the DABCO -catalyzed Rauhut–Currier reaction of α,β-unsaturated ketones and aryl acrylates: a computational inve ... - RSC Advances (RSC Publishing) DOI:10.1039/C6RA25311C
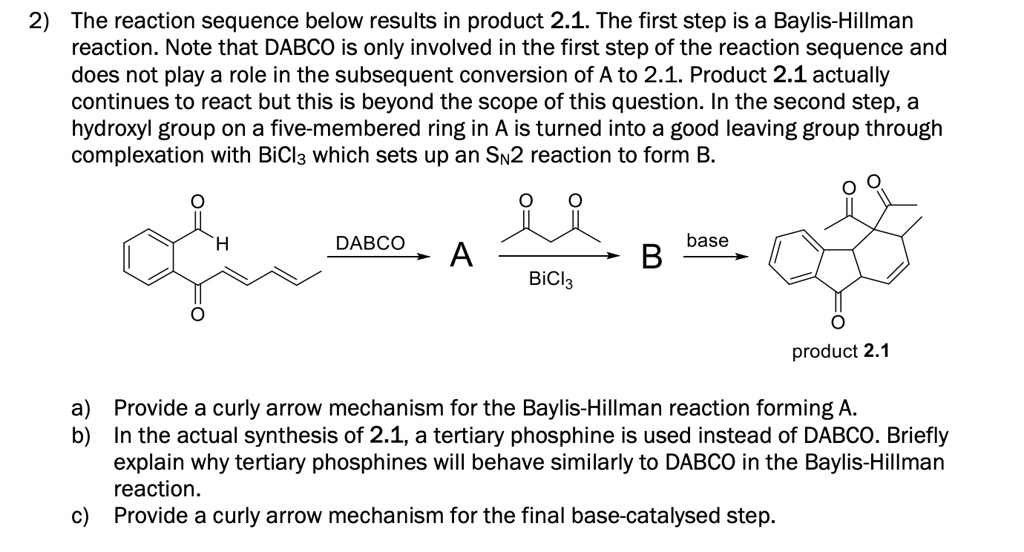
SOLVED:2) The reaction sequence below results in product 2.1_ The first step is a Baylis-Hillman reaction. Note that DABCO is only involved in the first step of the reaction sequence and does

Theoretical study on DABCO-catalyzed ring expansion of cyclopropyl ketone: Mechanism, chemoselectivity, and role of catalyst - ScienceDirect
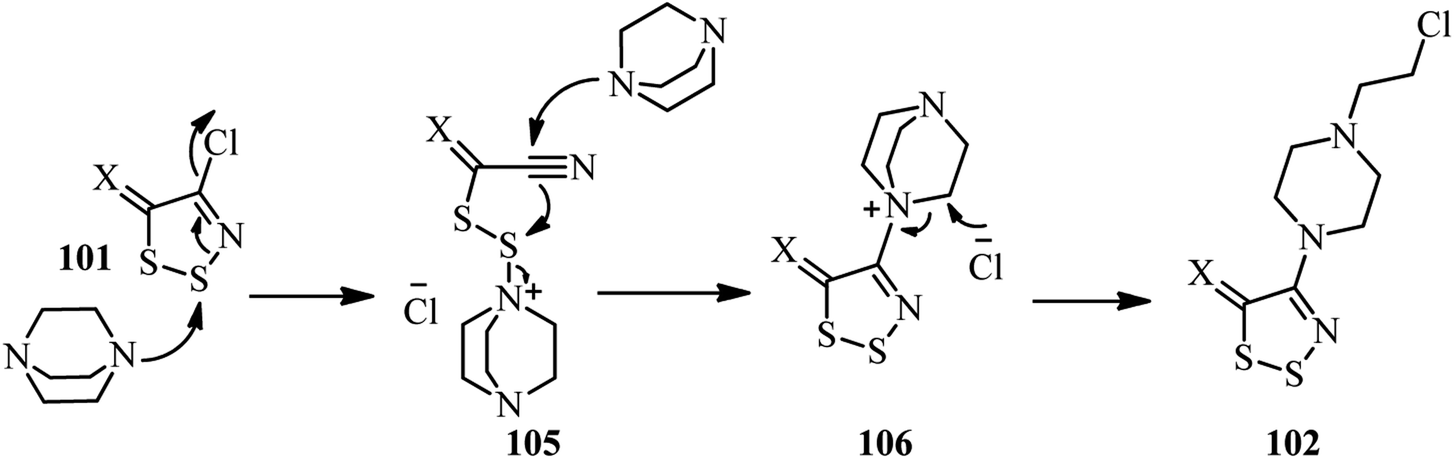
DABCO bond cleavage for the synthesis of piperazine derivatives - RSC Advances (RSC Publishing) DOI:10.1039/C9RA07870C

Exploration of the mechanism and scope of the CuI/DABCO catalysed CS coupling reaction - ScienceDirect

The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink
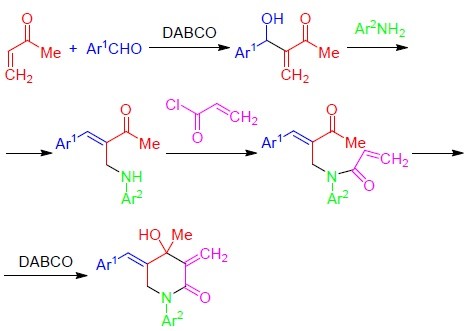
![Solved OH Ref. [52] O OH 0 0 4.21 DABCO H,CO OCHs OH 1.1.5 | Chegg.com Solved OH Ref. [52] O OH 0 0 4.21 DABCO H,CO OCHs OH 1.1.5 | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F607%2F6079c17b-d40e-440c-ab63-4860d1fa7c2a%2FphpbtT4hx.png)